Water Softener Effects on Chemistry and Flower Food
Research Update August 2024, powered by FloraLife
Garry Legnani, Ph.D. – Senior Postharvest Scientist, Smithers Oasis Company

Introduction:
Hard water, defined as having high concentrations of calcium and magnesium is a challenge for many of our customers. Poorly soluble calcium magnesium carbonates cause mineral deposits to accumulate in pipes, sinks, buckets, and vases. Wholesalers and bouquet manufacturers that go through high volumes of water daily often install expensive water treatment systems to mitigate hard water. These systems usually involve dilution of the hard water with reverse osmosis (RO) water to reduce the hardness to manageable levels; however, many florists and supermarket floral shops may have water softeners installed to prevent mineral deposits. There is a lot of misinformation regarding how water softeners affect water chemistry and little information on the effects of softened water on cut flowers, and the interaction with flower food. In this research update we will examine the effects of softened water on water chemistry parameters (hardness, alkalinity, pH, and conductivity) with and without the addition of flower food.
Water chemistry terms:
First, we want to review some water chemistry terms. For a more detailed look at water chemistry and how it relates to cut flower post-harvest care, we encourage you to read some of our past research updates available on our website. (RU Effects of Water Quality on Cut Flower Hydration and Flower Food Solutions)
pH – Potential of hydrogen is a logarithmic scale that measures the hydrogen ion concentration [H+] in a solution. The pH scale goes from 0 to 14 with pH 7 considered neutral, pH < 7 considered acidic, and a pH > 7 considered basic or alkaline. Because it is a logarithmic scale, a difference in one pH unit represents a 10-fold increase or decrease in [H+] concentration.
Hardness – The concentration of calcium [Ca2+] and magnesium [Mg2+] in the water. These minerals combine with carbonates to form poorly soluble calcium magnesium carbonate that result in mineral deposits at high concentrations.
Alkalinity – The concentration of carbonate [CO32-] and bicarbonate [HCO3-] in the water. Not to be confused with the term “alkaline” which is used to describe a solution with a pH > 7. If your water is high in carbonates and bicarbonates it will resist or “buffer” the effects of acids to lower the pH of your post-harvest treatment solutions. The higher the carbonate and bicarbonate concentration the more acid required to lower the pH. Alkalinity is measured in parts per million (ppm) calcium carbonate.
Conductivity – Electrical conductivity is related to the concentration of soluble salts in the water. Pure distilled water that contains no soluble salts will have a conductivity of zero and will not conduct electricity. Electrical conductivity units are in millisiemens/centimeter (mS/cm) and can be converted to ppm of total dissolved solids (TDS). Reverse osmosis (RO) systems use a selective membrane to remove almost all soluble salts from water.
Water softeners and how they work:
Water softeners work on a principle called ion exchange. Most softening systems consist of a tank containing a brine solution of sodium chloride and a cylinder packed with a resin that is charged with sodium [Na+]. Although less common, some softeners use potassium chloride. When hard water is circulated over the resin, the calcium and magnesium ions attach themselves to the resin, but to do so they must displace sodium ions. The calcium and magnesium in the water is therefore “exchanged” for sodium, preventing poorly soluble calcium magnesium salts from forming. The carbonate and bicarbonates remain in the water and combine with sodium. The sodium carbonate and bicarbonate that form is freely soluble in water. Because the carbonate and bicarbonates remain, the alkalinity of the water remains unchanged or may slightly increase. Two sodium ions are required to exchange with one calcium or magnesium ion so the conductivity of the water can also increase. The following illustrates the process of ion exchange:
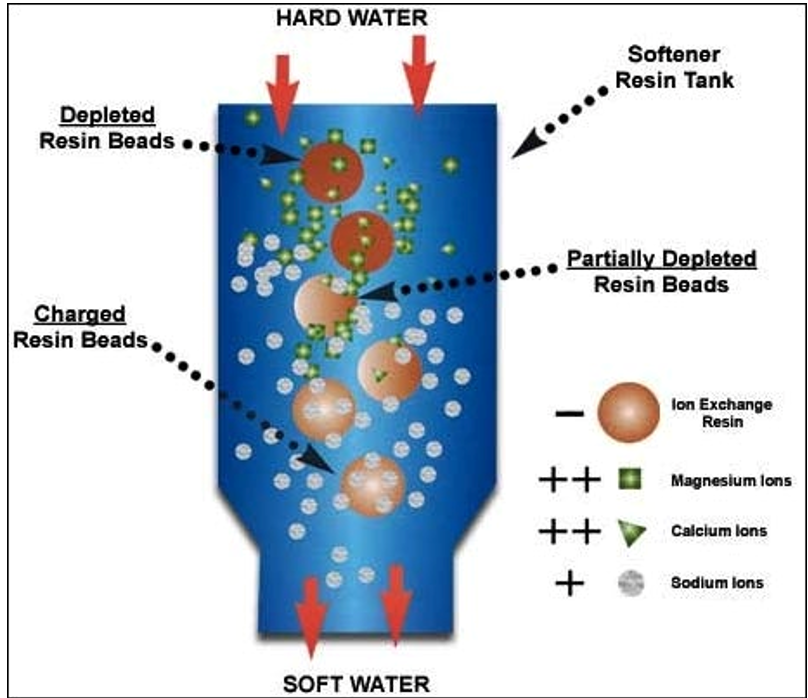
Source: https://medium.com/@thermodyneboilers/how-does-a-water-softener-work-4fc43bd9590b
Eventually the resin becomes saturated with calcium and magnesium and must be regenerated by flushing with brine. This regeneration cycle is commonly programed to occur based on water usage; however, it may also be programmed to occur in the early morning when water usage is low. When the system is regenerating, conductivity levels of your tap water can spike as salts are flushed into the system. The water will taste salty. Eventually these elevated salt levels return to normal after the regeneration cycle is completed.
Methods:
We took the following water samples from a softening system and measured hardness, alkalinity, pH, and conductivity.
- Untreated water – taken from a spigot/valve bypassing the softener
- Softened water – taken from the tap during normal softener operation
- 1:1 Untreated water: RO water
We then added flower food (FloraLife® Express 300) to the samples and remeasured the water quality parameters.
The following test kits and equipment were used to measure water quality parameters:
- Hach Total Hardness Test Kit Model 5-B
- LaMotte Total Alkalinity Test Kit – Titrator
- Thermo Scientific Orion 2 Star pH Meter with Orion pH SJ Gel Electrode
- Thermo Scientific 3 Star Portable Conductivity Meter
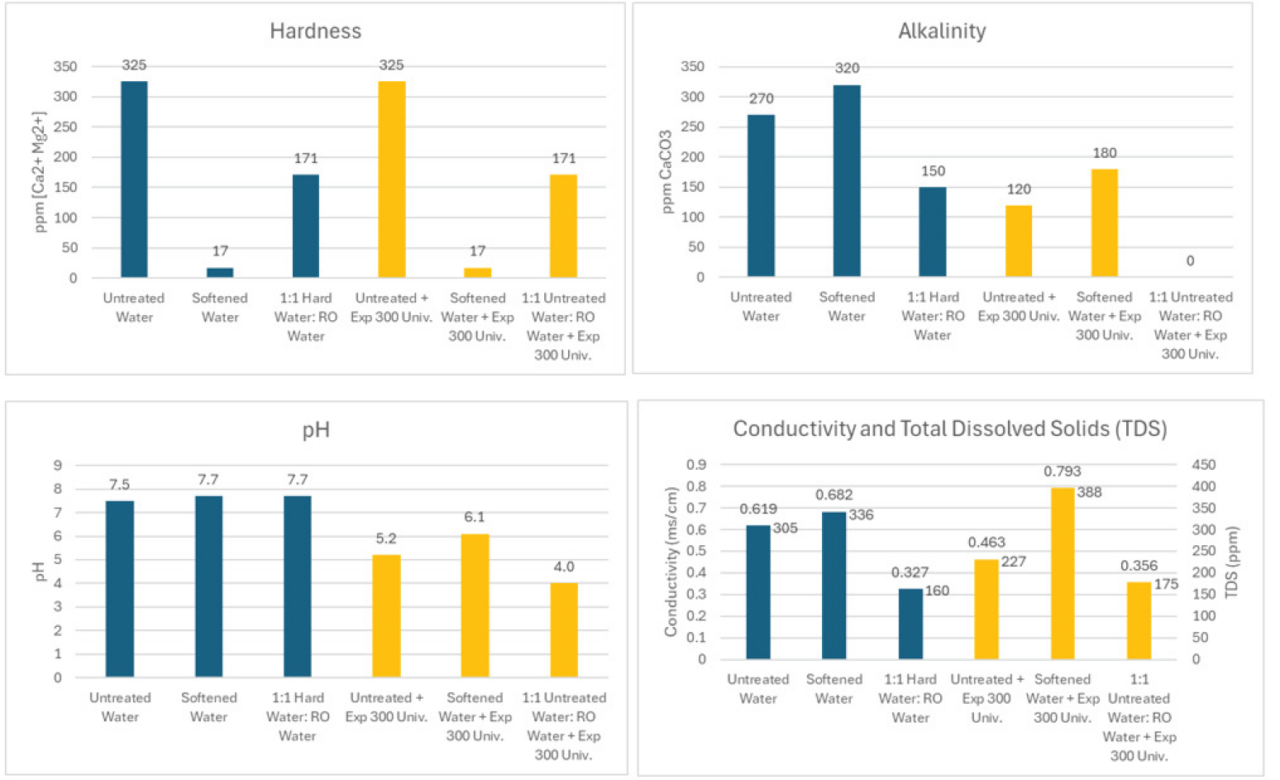
The untreated water had high hardness (325 ppm) and alkalinity (270 ppm). Following softener treatment, the hardness was reduced to 17 ppm, but alkalinity and conductivity levels increased. The increase in alkalinity and conductivity may be due to increased solubility of sodium carbonate compared to calcium carbonate, but we are just speculating. Diluting the hard water with equal parts RO water reduced hardness, alkalinity, and conductivity to moderate levels. pH was relatively unchanged in the three samples.
When flower food was added to the different water samples the untreated water showed no change in hardness, but the alkalinity was reduced by 55% from the acidifier reacting with the carbonates. The pH was reduced to 5.2 and conductivity was lower. When flower food was added to softened water, the hardness remained at 17 ppm, but the pH, alkalinity, and conductivity was higher compared to untreated water. When flower food was added to the 1:1 untreated water: RO water, hardness remained unchanged, and alkalinity was reduced to zero. pH was reduced to 4.0 and conductivity remained unchanged.
Note: We sampled water from the tap when the softener was going through its regeneration cycle. We observed a 6X increase in conductivity to 4.06 mS/cm or 1,988 ppm total dissolved solids (TDS), a level that would be phytotoxic to flowers.
How will softened water affect my flowers?
Calcium and magnesium are beneficial nutrients for cut flowers. Exchanging these for sodium will likely not harm cut flowers during normal softener operation (we are conducting flower testing to confirm this). However, we have observed that if softened water is used during the regeneration cycle, the high level of soluble salts caused foliar toxicity to roses. These toxicity symptoms appeared on the foliage as edge burn. In addition, softener treatment does not reduce alkalinity, reducing the effectiveness of the acidifier in flower food.

Foliar toxicity on roses observed when using softener water during regeneration cycle
Conclusions:
- Water softeners reduce hardness by exchanging calcium and magnesium for sodium.
- Water softeners do not affect pH.
- Water softeners do not reduce alkalinity but can increase it – this will reduce the effectiveness of the acidifier in the flower food and the pH of your flower food solution.
- Using softener water during the regeneration cycle will result in a spike in soluble salts which can be toxic to cut flower foliage.
